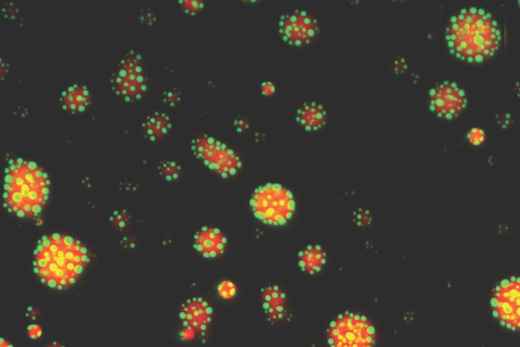
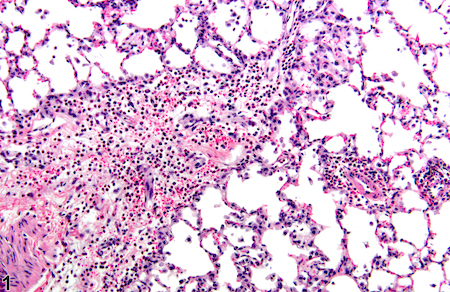
A relatively new imaging technique called photoacoustic imaging or PAI detects sounds produced when laser light interacts with human tissues. Working with colleagues at Michigan State, Emory immunologist Eliver Ghosn’s lab is taking the technique to the next step to visualize immune cells within atherosclerotic plaques.
The goal is to more accurately spot vulnerable plaque, or the problem areas lurking within arteries that lead to clots, and in turn heart attacks and strokes. A description of the technology was recently published in Advanced Functional Materials.
“I believe we are now closer to developing a more precise method to diagnose and treat life-threatening atherosclerotic plaques,” Ghosn says. “Our method could be deployed in combination with IVUS to significantly improve its accuracy and sensitivity, or it could be used non-invasively.”
Earlier this year, the FDA approved a photoacoustic imaging system for detection of breast cancer. Several companies are developing photoacoustic imaging systems, and what we might call “plain vanilla” PAI is currently being tested on carotid artery plaque in clinical studies in Europe.
Ghosn’s approach, developed with biomedical engineer Bryan Smith at Michigan State, adds specificity by adding nanoparticle probes taken up by macrophages, the immune cells that accumulate within atherosclerotic plaques. The nanoparticles, administered before imaging, act as contrast agents.