A combination immunotherapy of IL-21 and IFN-alpha, when added to antiviral therapy, is effective in generating highly functional natural killer cells that can help control and reduce SIV (simian immunodeficiency virus) in animal models. This finding, from Yerkes National Primate Research Center scientists in collaboration with Institut Pasteur, could be key for developing additional treatment options to control HIV/AIDS.
The results were published in Nature Communications.
Antiviral therapy (ART) is the current leading treatment for HIV/AIDS, and is capable of reducing the virus to undetectable levels, but is not a cure and is hampered by issues such as cost, adherence to medication treatment plan and social stigma.
To reduce reliance on ART, the Yerkes, Emory and Institut Pasteur research team worked with 16 SIV-positive, ART-treated rhesus macaques. In most nonhuman primates (NHPs), including rhesus macaques, untreated SIV infection progresses to AIDS-like disease and generates natural killer (NK) cells with impaired functionality. In contrast, natural primate hosts of SIV do not progress to AIDS-like disease. Determining why natural hosts do not progress or how to stop the progression is a critical step in halting HIV in humans.
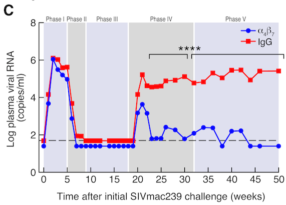
The researchers compared ART-only treated animals with animals that received ART, IL-21 and IFN-alpha to evaluate how the ART plus combination immunotherapy affected the amount of virus in the animals’ tissues.
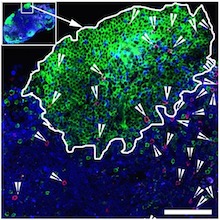
“Our results indicate ART plus combo-treated rhesus monkeys showed enhanced antiviral NK cell responses,” says first author Justin Harper, PhD, a senior research specialist and manager of the Paiardini research lab. “These robust NK cell responses helped clear cells in the lymph nodes, which are known for harboring the virus and enabling its replication and, therefore, the virus’ persistence. Targeting areas where the virus seeks refuge and knowing how to limit replication facilitate controlling HIV.”
HIV treatment has historically focused on the role of T cells in immunity, so harnessing NK cells opens up different avenues.
“This proof-of-concept study in rhesus monkeys, which progress to AIDS-like disease in the absence of ART, demonstrates how certain NK cell activities can contribute to controlling the virus,” says Mirko Paiardini, PhD, an associate professor of pathology and laboratory Medicine at Emory University and a researcher at Yerkes. “This opens the door to designing additional treatment strategies to induce SIV and HIV remission in the absence of ART, and, ultimately, reducing the burden HIV is to individuals, families and the world.”








![MM90301-10JK006-[RAW]](http://www.emoryhealthsciblog.com/wp-content/uploads/2010/11/MM90301-10JK006-RAW-300x214.jpg)