Scientists have identified a pair of molecules critical for T cells, part of the immune system, to travel to and populate the lungs. A potential application could be strengthening vaccines against respiratory pathogens such as influenza.
The findings were published online Thursday, September 26 in Journal of Experimental Medicine.

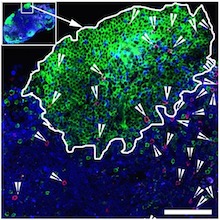
T cells in the lungs, courtesy of Alex Wein. Blue represents respiratory epithelium (EpCAM), while various T cells stain red, yellow or green.
Much research on immunity to influenza virus focuses on antibodies, infection- or vaccine-induced proteins in the blood that can smother viruses. But CD8 T cells, which survey other cells for signs of viral infection and kill infected cells, are an important arm of our defenses too. The epitopes – or bits of viral protein – they recognize generally do not change from year to year.
Researchers led by Jacob Kohlmeier, PhD, at Emory University School of Medicine wanted to learn more about what’s needed to get CD8 T cells into the lungs, since the lungs will often contain the first cells incoming virus will have a chance to infect. However, T cells don’t stick around in the lungs for extended amounts of time.
“The airways are a unique environment in the body,” says Alex Wein, a MD/PhD student who trained in Kohlmeier’s lab. “They’re high in oxygen but low in nutrients. Unlike other tissues, when T cells enter the airways, it’s a one-way trip and they have a half-life of a few weeks, so they must be continually repopulated.”
Wein, his fellow MD/PhD Sean McMaster, now at Boston Consulting Group, and Shiki Takamura at Kindai University are co-first authors of the paper. Kohlmeier is assistant professor of microbiology and immunology and part of the Emory-UGA Center of Excellence for Influenza Research and Surveillance.
The researchers showed that two molecules, called CXCR6 and CXCL16, are needed for CD8 T cells to reach the airways in mice. CXCR6 is found on T cells and CXCL16 is produced by the epithelial cells lining the airways of the lungs. Read more