Immunotherapies have transformed the treatment of several types of cancer over the last decade. Yet they focus on reactivating one arm of the immune system: cytotoxic T cells, which sniff out and kill tumor cells.
In a new paper in Nature, scientists at Emory Vaccine Center and Winship Cancer Institute of Emory University (Winship) report on their detailed look at B cells’ presence inside tumors. B cells represent the other major arm of the adaptive immune system, besides T cells, and could offer opportunities for new treatments against some kinds of cancers.
“Intratumoral B cells are an area of growing interest, because several studies have now shown that they are associated with a better prognosis and longer survival,” says first author Andreas Wieland, PhD, an Instructor in Rafi Ahmed’s lab at Emory Vaccine Center. “However, nobody really knows what those B cells are specific for.”
Wieland, Ahmed and colleagues decided to concentrate on head and neck cancers that were positive for human papillomavirus (HPV), because the virus provided a defined set of tumor-associated antigens, facilitating the study of tumor-specific B cells across patients.
“Our findings open the door for harnessing this type of cancer-specific immunity in future immunotherapy applications,” says Nabil Saba, MD, director of the head and neck medical oncology program at Winship. “This has implications not just for HPV-related squamous cell carcinomas of the head and neck, but for the broader field of immuno-oncology.”
The Emory Vaccine Center researchers worked with Saba and Winship surgeon Mihir Patel, MD to obtain samples of head and neck tumors removed from 43 patients.
“This has been a wonderful collaborative effort,” Patel adds. “We’re grateful to the patients whose tumor samples contributed to this study, and I’m looking forward to where this information takes us.”
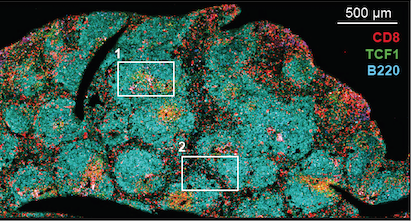
Within HPV-positive tumors, researchers found an enrichment for B cells specific to HPV proteins, and a subset of these cells were actively secreting HPV-specific antibodies. In the tumors, they could see germinal center-like structures, resembling the regions within lymph nodes where B cells are “trained” during an immune response.







 Those drugs are now FDA-approved for several types of cancer, yet some types of tumors do not respond to them. Studying exhausted CD8 T cells can help us understand how to better draw the immune system into action against cancer or chronic infections.
Those drugs are now FDA-approved for several types of cancer, yet some types of tumors do not respond to them. Studying exhausted CD8 T cells can help us understand how to better draw the immune system into action against cancer or chronic infections.