There’s a bit of sugar attached to your billion-dollar biotech product. Omitting the sugar (fucose) can help the product work better, Emory immunologists think.
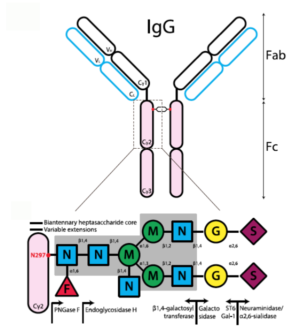
Fucosylation is the red triangle on this diagram of the carbohydrate modifications of antibodies. Adapted from KTC Shade + RM Anthony, Antibodies (2013) and used through Creative Commons license.
Many drugs now used to treat cancer and autoimmune diseases are antibodies, originally derived from the immune system. A classic example of a “therapeutic antibody” is rituximab, a treatment for B cell malignancies that was FDA-approved in 1997. It has been responsible for billions of dollars in revenue for its maker, pharmaceutical giant Roche.
Researchers at Emory Vaccine Center previously observed that in a mouse model of chronic viral infection, a traffic jam inside the body limits how effective therapeutic antibodies can be. One of the ways these antibodies work is to grab onto malignant or inflammatory cells. One end of the antibody is supposed to bind the target cell, while another is a flag for other cells to eliminate the target cell. During a chronic viral infection, a mouse’s immune system is producing its own antibodies against the virus, which form complexes with viral proteins. These immune complexes prevented the injected antibodies from depleting their target cells.
In a recent Science Immunology paper, postdoc Andreas Wieland, Vaccine Center director Rafi Ahmed and colleagues showed that antibodies that lack fucosylation have an enhanced ability to get rid of their intended targets. Fucosylation is a type of sugar modification of the antibody. (It is the red triangle in the diagram, provided by Wieland.) When it is not present, then the “flag for removal” region of the antibody can interact more avidly with the Fc gamma receptor on immune cells. Thus, the introduced antibodies can compete more effectively with the antibodies being produced by the body already.




