The study of human genetics has often focused on mutations that cause disease. When it comes to genetic variations in healthy people, scientists knew they were out there, but didn’t have a full picture of their extent. That is changing with the emergence of resources such as the Exome Aggregation Consortium or ExAC, which combines sequences for the protein-coding parts of the genome from more than 60,000 people into a database that continues to expand.

Rare mutations in the NMDA receptor genes cause epilepsy (GRIN2A) or intellectual disability (GRIN2B). Shown in blue are agonist binding domains of the receptors, where several disease-causing mutations can be found.
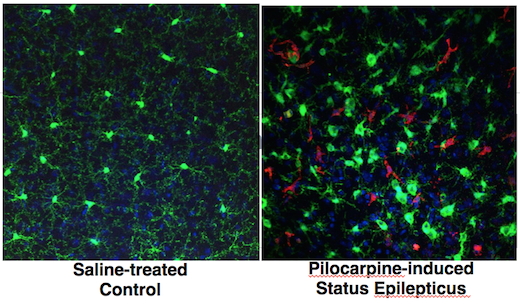
At Emory, the labs of Stephen Traynelis and Hongjie Yuan have published an analysis of ExAC data, focusing on the genes encoding two NMDA receptor subunits, GRIN2A and GRIN2B. These receptors are central to signaling between brain cells, and rare mutations in the corresponding genes cause epilepsy (GRIN2A) or intellectual disability (GRIN2B). GRIN2B mutations have also been linked with autism spectrum disorder.

Steve Traynelis and Hongjie Yuan
The new paper in the American Journal of Human Genetics makes a deep dive into ExAC data to explore the link between normal variation in the healthy population and regions of the proteins that harbor disease-causing mutations.
In addition, the paper provides a detailed look at how 25 mutations that were identified in individuals with neurologic disease actually affect the receptors. For some patients, this insight could potentially guide anticonvulsant treatment with a repurposed Alzheimer’s medication. Also included are three new mutations from patients identified by whole exome sequencing, one in GRIN2A and two in GRIN2B.
“This is one of the first analyses like this, where we’re mapping the spectrum of variation in a gene onto the structure of the corresponding protein,” says Traynelis, PhD, professor of pharmacology at Emory University School of Medicine. “We’re able to see that the disease mutations cluster where variation among the healthy population disappears.”

Heat map of agonist binding domain for GRIN2A. From Swanger et al AJHG (2016).
Postdoctoral fellow Sharon Swanger, PhD is first author of the paper, and Yuan, MD, PhD, assistant professor of pharmacology, is co-senior author.
It’s not always obvious, looking at the sequence of a given mutation, how it’s going to affect NMDA receptor function. Only introducing the altered gene into cells and studying protein function in the lab provides that information, Traynelis says.
NMDA receptors are complicated machines: mutations can affect how well they bind their ligands (glutamate and glycine), how they open and shut, or how they are processed onto the cell surface. On top of that complexity, mutations that make the receptors either stronger or weaker can both lead the brain into difficulty; within each gene, both types of mutation are associated with similar disorders. With some GRIN2A mutations, the functional changes identified in the lab were quite strong, but the effect on the brain was less dramatic (mild intellectual disability or speech disorder), suggesting that other genetic factors contribute to outcomes.
Clinical relevance
Traynelis and Yuan previously collaborated with the NIH’s Undiagnosed Disease Program to show that the Alzheimer’s medication memantine can be repurposed as an anticonvulsant, for a child with intractable epilepsy coming from a mutation in the GRIN2A gene. (Nature Communications, Annals of Clinical and Translational Neurology)
Memantine is an NMDA receptor antagonist, aimed at counteracting the overactivation of the receptor caused by the mutation. Memantine has also been used to treat children with epilepsy associated with mutations in the related GRIN2D gene. However, memantine doesn’t work on all activating mutations, and could have effects on the unmutated NMDA receptors in the brain as well. Traynelis reports that his clinical colleagues are developing guidelines for physicians on the use of memantine for children with GRIN gene mutations.
This study and related investigations were supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD082373), the National Institute of Neurological Disorders and Stroke (R24NS092989), the Atlanta Clinical & Translational Science Institute (UL1TR000454), and CURE Epilepsy: Citizens United for Research in Epilepsy.