The Alzheimer’s field has been in a “back to the basics” mode lately. Much research has focused on beta-amyloid, the toxic protein fragment that accumulates in plaques in the brain. Yet drugs that target beta-amyloid have mostly been disappointing in clinical trials.
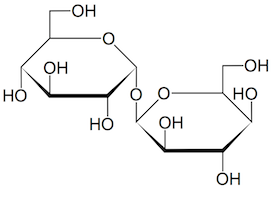
To broaden scope and gain new insights into the biology of Alzheimer’s, Emory investigators have been making large-scale efforts to catalog alterations of brain proteins. One recent example: Nick Seyfried and Erik Johnson’s enormous collection of proteomics data, published this spring in Nature Medicine. Another can be seen in the systematic mapping of N-glycosylation, just published in Science Advances by pharmacologist Lian Li and colleagues.
“It is very exciting to see, for the first time, the landscape of protein N-glycosylation changes in Alzheimer’s brain,” Li says. “Our results suggest that the N-glycosylation changes may contribute to brain malfunction in Alzheimer’s patients. We believe that targeting N-glycosylation may provide a new opportunity to help combat this devastating dementia.”