New research from Emory University indicates that nearly all people hospitalized with COVID-19 develop virus-neutralizing antibodies within six days of testing positive. The findings will be key in helping researchers understand protective immunity against SARS-CoV-2 and in informing vaccine development.
The test that Emory researchers developed also could help determine whether convalescent plasma from COVID-19 survivors can provide immunity to others, and which donors’ plasma should be used.
The antibody test developed by Emory and validated with samples from diagnosed patients has demonstrated that not all antibody tests are created equal – and that neutralizing antibodies, which provide immunity, have specific characteristics. Emory’s study focused on those neutralizing antibodies, which can stop the virus from infecting other cells.
The findings are now available on MedRxiv, the preprint server for health sciences, and are not yet peer-reviewed.
In the study, researchers looked at antibodies against the receptor-binding domain (RBD), part of the spike protein on the outside of the virus. The RBD is what grips on to human cells and allows the virus to enter them. The researchers focused on antibodies against the RBD because the sequence of the RBD in SARS-CoV-2 distinguishes it from other coronaviruses that cause the common cold.
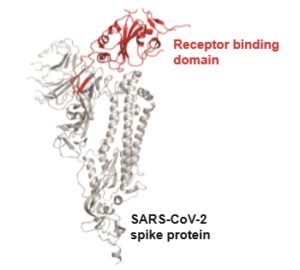
The receptor-binding domain, or RBD, is what grips on to human cells and allows the virus to enter them.
The initial 44 patient blood samples used in this study were from patients being treated for COVID-19 at Emory University Hospital and Emory University Hospital Midtown.
“These findings have important implications for our understanding of protective immunity against SARS-CoV-2, the use of immune plasma as a therapy, and the development of much-needed vaccines,” says Mehul S. Suthar, PhD, co-lead author and assistant professor of pediatrics at Emory University School of Medicine and Emory Vaccine Center. This study serves as the initial step in a much larger serology effort.




