Scientists at Winship Cancer Institute, Emory University have identified compounds that stop two elusive anticancer targets from working together. In addition to striking two birds with one stone, this research could expand the envelope of what is considered “druggable.”
 Many of the proteins and genes that have critical roles in cancer cell growth and survival have been conventionally thought of as undruggable. That’s because they’re inside the cell and aren’t enzymes, for which chemists have well-developed sabotage strategies.
Many of the proteins and genes that have critical roles in cancer cell growth and survival have been conventionally thought of as undruggable. That’s because they’re inside the cell and aren’t enzymes, for which chemists have well-developed sabotage strategies.
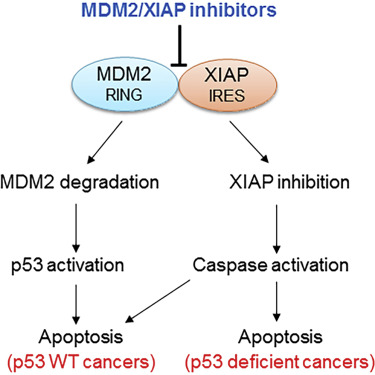
In a twist, the potential anticancer drugs described in Cancer Cell disable an interaction between a notorious cancer-driving protein, MDM2, and a RNA encoding a radiation-resistance factor, XIAP.
The compounds could be effective against several types of cancer, says senior author Muxiang Zhou, MD, professor of pediatrics (hematology/oncology) at Emory University School of Medicine and Aflac Cancer and Blood Disorders Center.
In the paper, the compounds show activity against leukemia and neuroblastoma cells in culture and in mice, but a fraction of many other cancers, such as breast cancers (15 percent) and sarcoma (20 percent), show high levels of MDM2 and should be susceptible to them.






