The immune system establishes “forward operating bases”, or lymph node-like structures, inside the tumors of some patients with kidney and other urologic cancers, researchers at Winship Cancer Institute of Emory University have discovered.

From left to right: Carey Jansen, Nataliya Prokhnevska, Hadyn Kissick and Viraj Master
Patients with well-supported immune cells in their tumors are more likely to control their cancers’ growth for a longer time — findings that could guide treatment decisions after surgery for kidney cancer. In addition, ongoing work has found the observation is broadly applicable to many cancer types, and it could help researchers expand the dramatic but sparse benefits of cancer immunotherapy to more people.
The results were published Wednesday, Dec. 11 in Nature.
“We knew that if there are more T cells in a tumor, the patient is likely to respond better to cancer immunotherapy,” says lead author Haydn Kissick, PhD. “But we were looking at a more basic question: why do some tumors have lots of T cells in them, and others don’t?”
Kissick is assistant professor of urology and microbiology and immunology at Emory University School of Medicine, Emory Vaccine Center and Winship Cancer Institute. His lab collaborated with surgeons and oncologists at Winship to examine tumor samples removed from patients with kidney, prostate and bladder cancer.
CD8 T cells hunt down and eliminate intruders – in this case, cancer cells. In patients with high levels of CD8 T cells residing in their tumors, their immune systems appeared to be better trained to suppress cancer growth after surgery, when small numbers of cancer cells (micrometastases) may be lurking elsewhere in the body. The cancers of those who had lower levels of CD8 T cells tended to progress four times more quickly after surgery than those with higher levels.
The finding has important implications, says Viraj Master, MD, who performed most of the kidney cancer surgeries. In this situation, additional treatments are not performed unless or until kidney cancer reappears, says Master, who is Fray F. Marshall Chair and professor of urology at Emory University School of Medicine and Winship’s Director of Integrative Oncology and Survivorship.
“Even after potentially curative surgery for aggressive kidney cancers, a significant fraction of patients will experience cancer recurrence,” he says. “But with this information, we could predict more confidently that some people won’t need anything else, thus avoiding overtreatment. However, on the basis of these findings, for others who are at higher risk of recurrence, we could potentially scan at more regular intervals, and ideally, design adjuvant therapy trials.”
The findings also provide insights for scientists interested in how the immune system successfully controls some cancers, but with others, the T cells become increasingly exhausted and ineffective.
“This study may lead to new insights into why immunotherapy can be so effective in some cancer types, but rarely works in others such as prostate cancer, and may highlight a path forward for developing more effective immunotherapy treatments,” says Howard Soule, PhD, executive vice president and chief science officer for the Prostate Cancer Foundation, which supported the Winship team’s work.
Kissick and his colleagues were surprised to find “stem-like” T cells, or precursors of exhausted cells, inside tumor samples. Stem-like T cells are the ones that proliferate in response to cancer immunotherapy drugs, which can revive the immune system’s ability to fight the cancer.
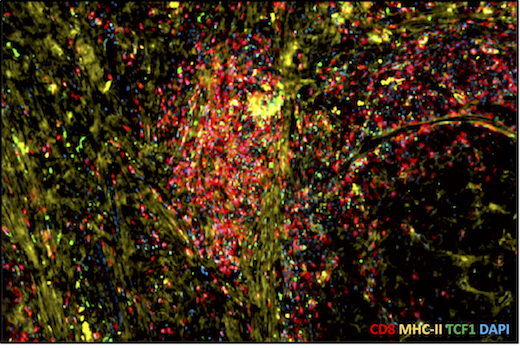
Tumor sample with high level of T cell infiltration. Red = CD8, yellow = MHC class II, a sign of APCs
“Lymph nodes are like ‘home base’ for the stem-like T cells,” says Carey Jansen, an MD/PhD student who is the first author of the Nature paper. “We had expected that the stem-like cells would stay in lymphoid tissue and deploy other T cells to infiltrate and fight the cancer. But instead, the immune system seems to set up an outpost, or a forward base, inside the tumor itself.”
The researchers found that other immune cells called “antigen-presenting cells” or APCs, which are usually found within lymph nodes, can also be seen within tumors. APCs help the T cells figure out when and what to attack. Like high numbers of CD8 T cells, high numbers of APCs in tumors were also a predictor of longer progression-free survival in kidney cancer patients.
The APCs and the stem-like cells were usually together within the same “nests,” in a way that resemble how the two types of cells interact in lymph nodes. This relationship was apparent in kidney cancers and also in samples from prostate and bladder cancers.
“The question of how the stem-like cells get into a tumor was not answered, but we do think that the APCs support the stem-like cells and are necessary for their maintenance,” Kissick says. “Given that these are the cells responsive to cancer immunotherapy agents, focusing on the relationship between the APCs and the T cells within the tumors could be valuable.”
Additional co-authors include: graduate student Nataliya Prokhnevska, urology chair Martin Sanda, MD and biostatistician Yuan Liu, PhD.
The research was supported by the National Cancer Institute (R00CA197891, U01CA113913), the Prostate Cancer Foundation, Swim Across America, the James M. Cox Foundation, James C. Kennedy, the Dunwoody Country Club Senior Men’s Association and an educational grant from Adaptive Technologies.














