Who should get stents, the tiny metal tubes designed to keep clogged coronary arteries open? Someone who is having a heart attack certainly should, and the life-prolonging benefits have been demonstrated in several studies. But results have been more ambiguous for patients who have “stable angina”: chest pain that comes with exertion but goes away at rest.

Kreton Mavromatis, MD
A recent study addressing this topic called FAME 2 has received extensive media coverage. It was published in the New England Journal of Medicine and also presented at the European Society of Cardiology meeting in Munich. Kreton Mavromatis, MD, director of cardiac catheterization at the Atlanta VA Medical Center and assistant professor of medicine at Emory, was a co-author on the NEJM paper.
In the new study, researchers used a technique called fractional flow reserve (FFR) to decide if someone with stable angina should get a stent, or receive medical therapy with drugs such as aspirin and statins. Conventionally, X-ray coronary angiography is used to assess the need for a stent.
FFR involves introducing a pressure sensor via guidewire into the coronary artery, to measure how much blood flow is being blocked. FAME 2 was sponsored by St Jude Medical, a company that makes guidewire equipment for use in FFR.
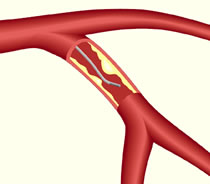
Fractional flow reserve is a way of assessing the effects of blockages in blood flow in a coronary artery.
The clinical trial was stopped early because of clear differences in the rates of hospitalization (4 percent for stents against 13 percent for medical therapy)
“FAME 2 showed that the strategy of treating stable ischemic heart disease with FFR-guided coronary stenting reduces the combination of death, MI and urgent revascularization as compared with strategy of medical therapy alone,” Mavromatis says. “This benefit was specifically due to the reduced need of urgent revascularization due to acute coronary syndrome, a dramatic event for our patients.”
Some cardiologists have criticized the FAME 2 study, noting that the benefits of stenting didn’t come in terms of reducing “hard events” (deaths and heart attacks).
“It is important to recognize that less symptoms of angina and less chance of hospitalization are tremendous benefits that our patients really appreciate,” Mavromatis says. “I think FFR will play a bigger role in evaluating and treating coronary artery disease, as it can direct stenting much more precisely than angiography toward clinically important coronary artery disease, improving patients’ outcomes and saving money.”
The FFR procedure costs several hundred dollars but that is significantly less than the cost of implanting a coronary stent. Habib Samady, MD, director of interventional cardiology at Emory, has also been an advocate for the use of FFR to select who would benefit from a coronary stent. He wrote an article describing its uses in 2009:
We have been using and advocating FFR since pressure guidewire technology first came to the U.S. in 1998. At Emory, we are sometimes asked to reevaluate patients who have been slated for CABG surgery at another hospital where recommendations are made based on angiography alone. When we evaluate these cases using FFR, we are sometimes able to recommend courses of treatment that involve fewer stents or even medical therapy. Occasionally, based on FFR data, we send our patients for an endoscopic or “minimally invasive” bypass and stent the remaining narrowings.
In addition, FFR has helped reduce the number of multi-vessel PCIs performed. Patients who might have received stents in three vessels after angiography alone would likely receive stents in only one or two vessels after FFR-guided analysis. Among patients with single-vessel disease, FFR often has allowed us to recommend medical treatment in lieu of stenting. Implanting fewer stents also means using less contrast agent and fewer materials, which lowers the expenses involved in treatment.
A large, multi-center study called ISCHEMIA is starting that will address the coronary stent vs medical therapy issue in a more definitive way. Both Emory and the Atlanta VA Medical Center are participating. “This is a very important next step in understanding the benefits of invasive therapy of stable ischemic heart disease,” Mavromatis says.