Loss of balance and falls are big concerns for people living with Parkinson’s disease and their caregivers. Researchers at Emory and Georgia Tech recently published a paper in PLOS ONE providing insights into how sensory and motor information are misrouted when people with Parkinson’s are attempting to adjust their balance.
When the researchers examined 44 people with Parkinson’s, their history of recent falls correlated with the presence and severity of abnormal muscle reactions. This could help clinicians predict whether someone is at high risk of falling and possibly monitor responses to therapeutic interventions.
People with Parkinson’s tend to lose their balance in situations when they are actively trying to control their center of mass, like when they are getting up from a chair or turning around. Disorganized sensorimotor signals cause muscles in the limbs to contract, such that both a muscle promoting a motion and its antagonist muscle are recruited. It’s like stepping on the gas and the brake at the same time, says J. Lucas McKay, who is first author of the paper.
Physical therapists are sometimes taught that balance reactions in Parkinson’s patients are slower than they should be.
“We show this is not true,” McKay says. “The reactions are on-time but disorganized.”
The paper extends groundbreaking work on how muscles maintain balance, conducted by co-author Lena Ting in animals and healthy young humans, to people with Parkinson’s. Co-authors of the PLOS One paper include Ting and Parkinson’s specialists Madeleine Hackney and Stewart Factor, director of Emory’s movement disorders program. McKay is assistant professor of neurology and biomedical informatics.
McKay says that sensorimotor problems may be a result of degeneration of regions of the brain, outside of and after the dopaminergic cells in the basal ganglia.
“We have to speculate, but the sensory misrouting would be occurring in brain regions like the thalamus — not usually the ones we think about in Parkinson’s, such as the basal ganglia,” he says. “This suggests that future therapies involving these areas could reduce falls.”
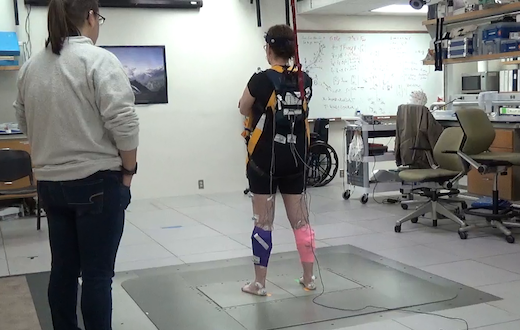
The set-up that researchers used to measure balance reactions resembles an earthquake simulator, and was designed and customized by Ting. The photo shows one of the Parkinson’s study participants, being watched by a physical therapy student.