Geneticist Tamara Caspary’s laboratory has a recent paper in the journal Development showing how a developing mammalian embryo can correct a mispatterned neural tube over time. Former Genetics + Molecular Biology graduate student Chen-Ying Su, now a postdoctoral fellow at the Fred Hutchinson Cancer Research Center in Seattle, is the first author of the paper.
A molecule called “Sonic Hedgehog†is needed for proper patterning of the brain, spinal cord and eyes – it provides signals to the cells in the embryo, telling them what to become. Mutations that enhance Sonic Hedgehog signaling can lead to neural tube defects, some of the most common birth defects in humans, while those that diminish it can lead to holoprosencephaly, malformations of the brain and face. However, the majority of neural tube defects such as spina bifida do not come solely as a result of genetics – doctors think that getting enough (and possibly, not too much) of the B vitamin folic acid can prevent most of them.

Red = motor neuron precursor, green = later motor neuron marker
Mutation of Arl13b causes expansion of motor neurons (B and J)
Later deletion causes temporary expansion (C), corrected two days later (K)
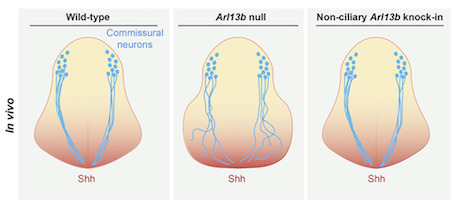
Su and her colleagues examined mouse development in a situation where patterning of the neural tube is disrupted for a short time, because of a deletion in a gene (Arl13b), which helps to carry out Sonic Hedgehog’s instructions.
If Arl13b is not working starting from the beginning of development, embryos have an expansion of motor neurons, at the expense of other types of cells. The mutation leads to an open neural tube as well as abnormal eye, heart and limb development. However, if the deletion of Arl13b occurs on the ninth day, the embryo can recover proper patterning over the next few days. Mouse pregnancies last roughly three weeks.
Caspary says that while the relationship between Hedgehog signaling and neural tube defects is complicated, her lab’s recent work “does help define the time window during which we could non-surgically correct neural tube defects in utero.â€
“In addition, it points to the importance of what we call “plasticity”- that cells can make incorrect decisions and correct them if still in a competency window, much like we think of adolescence,†she says. “It hints at the promise of stem cell research, that cells might be coaxed into other fates even though they start expressing tissue-specific markers. And it shows that the embryo is still much better at it than we are in a tissue culture dish.â€
Posted on
October 17, 2012 by
Quinn Eastman
in Uncategorized