Cardiac cell therapy sounds like a promising idea: use the patients’ own cells to enhance healing or even regenerate the damaged heart muscle. Doctors have taken up the promise, testing it in clinical trials involving thousands of patients. But a basic problem facing the field is this: naked cells don’t appear to stay in the heart or stay alive for long enough to provide a sustained benefit.
Three labs at Emory have published papers in the last year addressing this problem. All describe some kind of supportive biomaterials, consisting of capsules or a gel, which help cells stay put and stay alive, in experiments where recovery from a heart attack is modeled in rodents.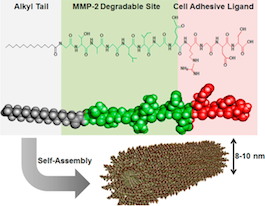
The most recent comes from cardiologist Young-sup Yoon and colleagues, in ACS Nano. The first author is Kiwon Ban, a senior postdoc in Yoon’s laboratory. Ban and his team use self-assembling peptides, developed in collaboration with biomaterials engineer Ho-wook Jun at UAB (see figure). The peptides form a gel that both physically keeps cardiac muscle cells in the heart and eases their integration into the heart tissue over a period of weeks. As Katie Bourzac explains in Chemical & Engineering News:
One peptide acts like a natural protein that adheres to cells and promotes cell survival. The second peptide is readily broken down by a protease. The team designed the gel so that when it is implanted, it begins to degrade a bit, allowing cells from the body to migrate in. Eventually the gel should disintegrate completely as the heart tissue builds its own extracellular matrix. This particular gel has already performed well as a support for other kinds of cells grown from stem cells, including pancreatic and muscle cells.
We thought it may be useful to readers to be able to compare and contrast these papers in chart form. Read more





