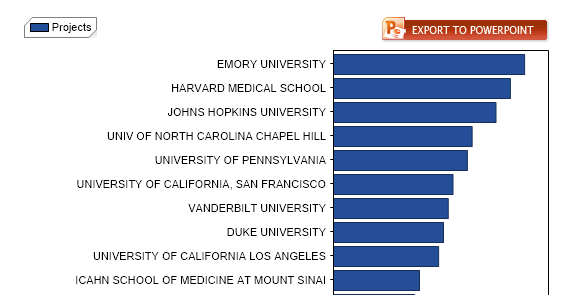
Emory scientists have identified a way to stop troublemaker cells that are linked to immune rejection after kidney transplant. The finding could eventually allow transplant patients to keep their new kidneys for as long as possible, without the side effects that come from some current options for controlling immune rejection.
The results are published in Journal of Clinical Investigation.
The standard drugs used for many years, calcineurin inhibitors, show side effects on cardiovascular health and can even damage the kidneys over time. A newer FDA-approved medication called belatacept, developed in part at Emory, avoids these harmful effects but is less effective at stopping acute rejection immediately after the transplant. Belatacept is a “costimulation blocker” – it interferes with a signal some immune cells (T cells) need to proliferate and become activated.
Researchers led by Emory transplant surgeon Andrew Adams, MD, PhD suspected that long-lasting memory CD8+ T cells were resistant to belatacept’s effects.
“Our previous work identified that memory CD8+ T cells may be elevated in animals and human patients who go on to reject their transplanted organs while taking belatacept,” says Dave Mathews, an MD/PhD student who worked with Adams and is the first author of the paper.
The researchers identified a certain marker, CD122, which was present on memory CD8+ T cells and important for their activity. On T cells, CD122 acts as a receiving dish for two other secreted molecules, IL-2 and IL-15, generally thought of as inflammatory cytokines, or protein messengers that can encourage graft rejection. Read more






 o discuss research projects. She is now examining potential treatments for opiate addiction based on galanin, a neuropeptide found in the brain.
o discuss research projects. She is now examining potential treatments for opiate addiction based on galanin, a neuropeptide found in the brain.