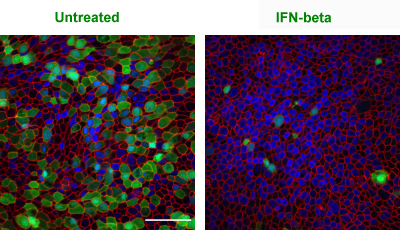
In severe cases of COVID-19, Emory researchers have been observing an exuberant activation of B cells, resembling acute flares in systemic lupus erythematosus (SLE), an autoimmune disease.
The findings point towards tests that could separate some COVID-19 patients who need immune-calming therapies from others who may not. It also may begin to explain why some people infected with SARS-CoV-2 produce abundant antibodies against the virus, yet experience poor outcomes.
The results were published online on Oct. 7 in Nature Immunology.
The Emory team’s results converge with recent findings by other investigators, who found that high inflammation in COVID-19 may disrupt the formation of germinal centers, structures in lymph nodes where antibody-producing cells are trained. The Emory group observed that B cell activation is moving ahead along an “extrafollicular” pathway outside germinal centers – looking similar to what they had observed in SLE.
Update: check out first author Matthew Woodruff’s commentary in The Conversation: “The autoimmune-like inflammatory responses my team discovered could simply reflect a ‘normal’ response to a viral infection already out of hand. However, even if this kind of response is ‘normal,’ it doesn’t mean that it’s not dangerous.”

Before the COVID-19 pandemic, co-senior author Ignacio (Iñaki) Sanz and his lab were focused on studying SLE and how the disease perturbs the development of B cells.
“We came in pretty unbiased,” Sanz says. “It wasn’t until the third or fourth ICU patient whose cells we analyzed, that we realized that we were seeing patterns highly reminiscent of acute flares in SLE.”
In people with SLE, B cells are abnormally activated and avoid the checks and balances that usually constrain them. That often leads to production of “autoantibodies” that react against cells in the body, causing symptoms such as fatigue, joint pain, skin rashes and kidney problems. Flares are times when the symptoms are worse.