A term we heard a bunch at the Emory Microbiome Symposium in November was “metagenomicsâ€. Time for an explainer, with some help from Emory geneticist Tim Read.
Nature Reviews Microbiology defines metagenomics as “genomic analysis of microbial DNA that is extracted directly from communities in environmental samples.â€
This technology — genomics on a huge scale — enables a survey of the different microorganisms present in a specific environment, such as water or soil, to be carried out. Metagenomics is also emerging as a tool for clinical diagnosis of infectious diseases.
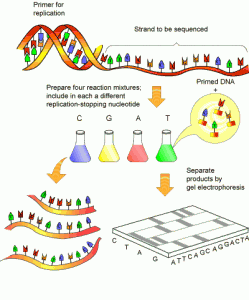
Read notes that the term specifically refers to “shotgun†sequencing of environmental DNA.
“The shotgun approach is to randomly sample small pieces of the DNA in the tube, no matter which organism they came from,†he says. “The output is a mélange of different genes from bacteria, viruses, fungi, plants and humans. The data is fascinating but the analysis is daunting.†Read more