Instead of complication-prone electronic cardiac pacemakers, biomedical engineers at Georgia Tech and Emory envision the creation of “biological pacemakers.” Hee Cheol Cho and colleagues have been taking advantage of his work on a gene called TBX18 that can reprogram heart muscle cells into specialized pacemaker cells.

Graduate student Sandra Grijalva in lab
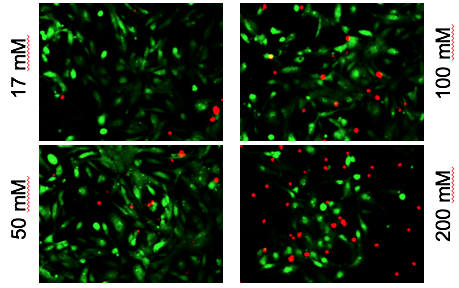
Every heartbeat originates from a small group of cells in the heart called the sinoatrial node. How these cells drive contractions in the relatively massive, and electrically sturdy, rest of the heart is a problem cardiology researchers call the “source-sink mismatch.” Until Cho’s innovations, it was only possible to isolate a handful of pacemaker cells from animal hearts, and the isolated cells could not be cultured.
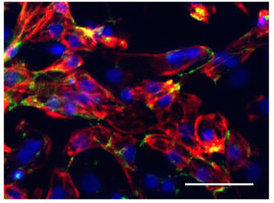
Cho and colleagues recently published a paper in Advanced Science describing TBX18-induced pacemaker cell spheroids, a platform for studying source-sink mismatch in culture
Graduate student Sandra Grijalva is the first author of the paper. We first spotted Grijalva’s work when it was presented at the American Heart Association meeting in 2017. Read more