The idea that doctors could use stem cells to treat diseases ranging from amyotrophic lateral sclerosis (ALS) to stroke, spinal cord injury and heart disease has stimulated excitement and research funding over the last decade.
One critical obstacle is getting the stem cells to survive in the harsh environment of injured tissue and turn into the right kind of cell where they are needed. In both laboratory experiments and clinical trials, most of the stem cells usually die a few days after transplantation.
Exposing stem cells to reduced levels of oxygen may actually help protect them from the stressful process of being transplanted into the heart, according to recent research.
Shan Ping Yu and Ling Wei, who moved their laboratories about a year ago to Emory’s Department of Anesthesiology, were the first to show the effects of “hypoxic preconditioning.” Wei says the low oxygen strategy is a continuation of previous collaboration with Comprehensive Neurosciences Center director Dennis Choi. There, they had used the tactic of overexpressing BCl2, a gene that counteracts cell death, but the new approach avoids permanently altering the genes in stem cells, which may have long-term adverse effects.
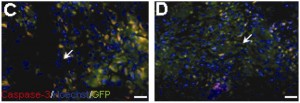
Effects on mesenchymal stem cells' ability to implant into heart tissue in rats. In D, the stem cells were exposed to low oxygen but in C they were not. Blue shows all cell nuclei, while green shows implanted stem cells. The greater presence of yellow in C, a couple days after transplantation, displays the activation of an enzyme that leads to cell death. From the Journal of Thoracic and Cardiovascular Surgery.
In a way, this is consistent with the work of former Emory investigator Marie Csete, who showed that stem cells are happier and healthier in oxygen concentrations that reflect the levels they experience in the body: between 2 and 5%.
To achieve their protective effects, Yu and Wei are using oxygen concentrations of 0.5%. For comparison, room air has about 20% oxygen.
In an editorial, Yu, Wei and graduate student Molly Ogle discuss how they have been exploring whether inhibitors of enzymes that sense levels of oxygen in cells could have the same protective effects as exposure to low oxygen. Yu also reports that his group is studying how low oxygen helps stem cells home to target tissues better. Their hypothesis is that low oxygen stimulates cells’ motility — their ability to migrate into the right place. Wei’s research has shown that lower oxygen helps more stem cells to turn into neuronal cells.